Struggling with Poor Solubility, Bioavailability, or CMC Hurdles?
Get Expert Guidance from Catalent Nottingham Early Phase Development Team!
When solubility, bioavailability, or CMC setbacks threaten your timeline, Catalent Nottingham steps in with proven early-phase expertise, so you can move forward with confidence.
Book your free 1:1 Molecule Developability Assessment consultation, valued at £3,000
Our senior expert advisors will assess your molecule and map a smarter route to clinic, tailored to your goals and timeline using our proven methodology:
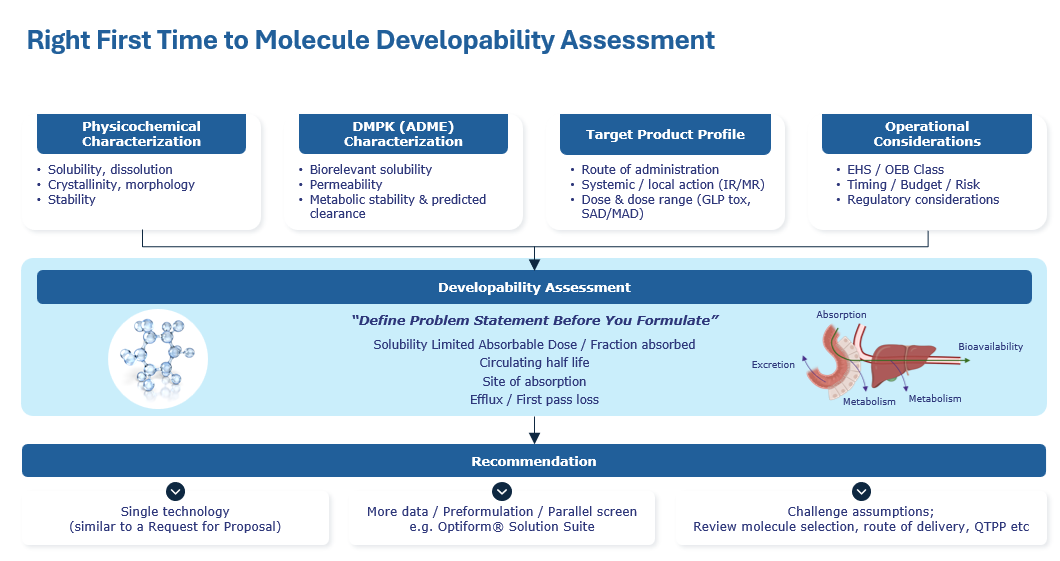
Limited availability. Secure your preferred consultation slot today.
DON’T LET DEVELOPMENT BOTTLENECKS DERAIL YOUR PROGRAM
Behind every delay is a decision that could’ve gone better, with the right partner. Catalent Nottingham is the Early Phase Center of Excellence in Europe that leading innovators trust to:
- Fix low solubility without sacrificing speed
- Maximize bioavailability with minimal API
- Navigate regulatory CMC requirements confidently
- Formulate for pediatric and orphan drug success
MEET YOUR ADVISORS
Get actionable insights from our senior team, trusted by global pharma innovators.
Stephen Tindal, Director, Scientific Advisory, Oral Small Molecules
Matt Ling, Ph.D., Director, Scientific Services
Brigida Allieri, Ph.D., Technical Manager and Scientific Advisor
WHY CATALENT NOTTINGHAM
With over 20 years of experience and a fully integrated facility, we help you move faster with fewer hurdles.
- MHRA- and FDA-approved GMP facility with full preclinical to small-scale commercial capabilities
- OEB 1–3 and controlled drug license
- Formulation development from simple to complex, including micronization, spray drying, HME, and analytical characterization
- End-to-end support from PBPK modeling through Phase 1 clinical trial material, with integrated analytical services.
- Phase 2, 3 and small-scale commercial manufacturing capabilities, with PBPK support.
TRUSTED BY INNOVATORS ACROSS EUROPE
- “Your PBPK work has saved this molecule that was practically killed”
- “Your MDA has convinced us that you understand our needs and we will partner with you”
- “Huge thanks to the Catalent Nottingham team… we have completed a huge volume of work over a short space of time, and we really appreciate all the hard work you have put in”
- “Your team’s efforts on PBPK modeling has increased our confidence going in clinical studies”
- “Catalent Nottingham helped us reduce risk in our Phase 1 formulation with rapid turnaround and deep scientific insight”
BOOK YOUR CONSULTATION NOW
Complete the form below to connect with our expert advisors. We’ll reach out within 2 -3 business days to schedule your session.
