Zydis® Orally Disintegrating Tablets
Zydis® ODT (orally disintegrating tablet) is a fast-dissolving formulation that disperses instantly in the mouth without water. With more than 35 products launched in 60 countries, it continues to be the world’s best-in-class ODT technology.
Our expert team is the best trained in the world, offering feasibility evaluations as well as support across the entire lifecycle of your product.
Whether you are considering an ODT to enhance pharmacokinetics through pre-gastric absorption, looking for a way to improve patient compliance or seeking a marketing advantage for a valued brand, Zydis® ODT can help enhance the value of your investment and accelerate your product’s potential.
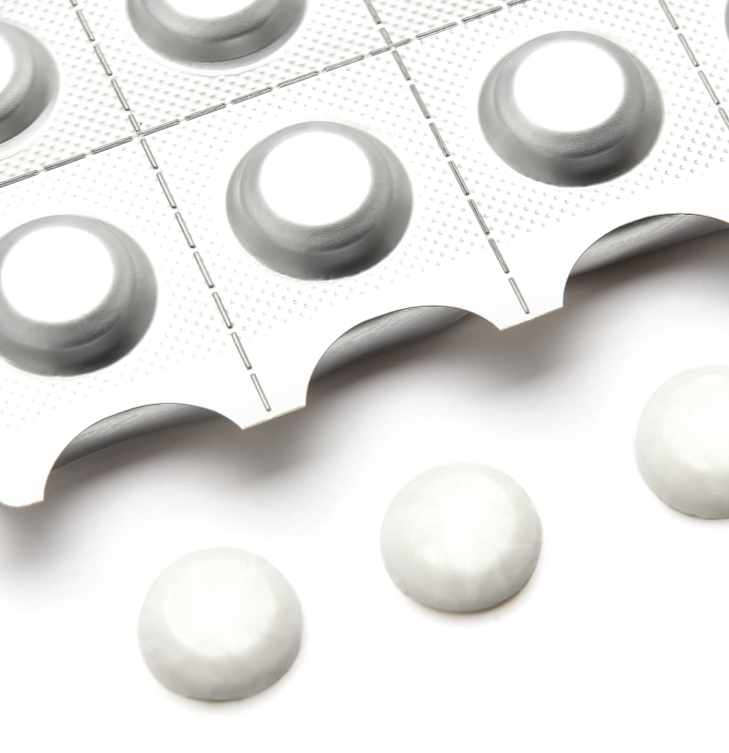
Capabilities & Expertise
Partner with our team of experts to accelerate your Zydis® ODT journey from concept to market. We offer a complete suite of services—from feasibility through full-scale commercial manufacturing—so you can bring innovative therapies to patients.
We start with a tailored feasibility program that meets your product’s specific requirements. Our process begins with a technical evaluation of preformulation data and considers your API’s unique characteristics. We then prepare a range of prototype Zydis® ODT formulations under different processing conditions at a bench scale. Using analytical techniques, we determine the compatibility of your candidate API with the Zydis® technology. After short-term (4-week) accelerated physical stability studies, we make recommendations for a full development program.
At our state-of-the-art Swindon, U.K. facility, we ensure a smooth transition from bench to pilot to large-scale cGMP manufacturing. With integrated expertise and seamless scale-up, we help you minimize risk, accelerate timelines and achieve commercial success.
Valuable Product Differentiation
- High level of customer preference & enhanced market appeal
- Embossing with corporate logos and product codes
- Rapid onset of action
- Unique packaging, including child-resistant options
- Multiple colors and shapes
- Taste masking and flavors formulated for specific markets, including pediatrics
Zydis® Bio
The Zydis® platform continues to evolve with innovations designed to meet the needs of today’s therapies and tomorrow’s breakthroughs. Zydis® Bio ODT extends the technology to biologics, enabling sublingual and buccal delivery of large molecule peptides, proteins, vaccines, and allergens while protecting drug integrity and ensuring patient convenience.
Key Benefits:
- Potential for sublingual / buccal absorption
- Solid, unit doses presented in protective pack
- Low temperature processing minimizes manufacturing losses of labile drugs
- Solution / suspension dosing achieves good content uniformity for low dose actives
- Solid dosage form and low water activity aids long term stability
- Liquid processing facilitates containment of potent drugs in production
Zydis® Bio is helping innovators unlock new therapeutic possibilities and expand treatment accessibility, and reinforces Zydis® as the world’s leading orally disintegrating tablet technology.
How It Works
Zydis® ODTs are made through a four-step process:
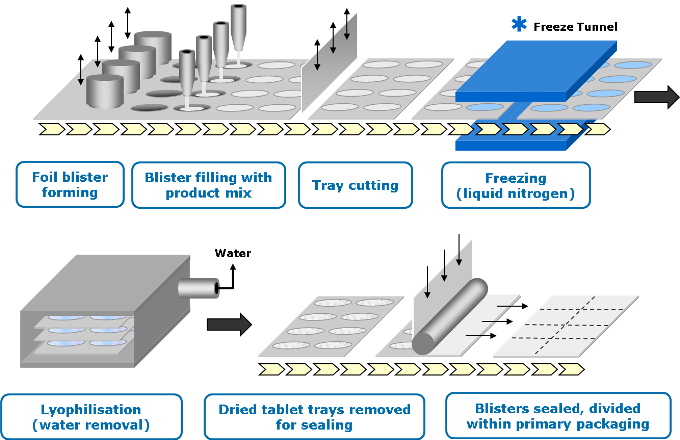
Mixing
The bulk API is formulated into a liquid solution or suspension.
Filling and freezing
The liquid is precisely filled into pre-formed blisters and passed through a specially designed cryogenic freezing process to control the ultimate size of the ice crystals.
Lyophilization
The frozen units are then transferred to large-scale freeze dryers for the lyophilization process.
Sealing
The blisters containing the dried Zydis® units are then sealed via a heat-seal process to protect the product from varying environmental conditions and ensure long-term stability.
Facilities

Swindon, U.K.
Catalent’s Swindon, U.K. facility is dedicated to Zydis® ODT development and manufacturing and produces more than 1.5 billion doses annually.
Swindon is subject to annual review to ensure the highest levels of compliance with global health authorities to guarantee every dose meets the most stringent standards for quality, safety and efficacy.
Ready to Fuel Your Mission?
Partner with Catalent to develop ODTs that redefine patient convenience and product performance. Our experts can help you deliver trusted, fast-dissolving tablets worldwide.





